Bioendo KC Endotoxin Test Kit (Kinetic Chromogenic Assay)
1. Product Information
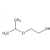
In BioendoTM KC Endotoxin Test Kit, Amebocyte Lysate is co-lyophilized with chromogenic substrate. Therefore, bacterial endotoxin could be quantified based on the chromogenic reaction. The assay is strong resistance to interference, and has advantages of kinetic turbidimetric and end-point chromogenic method. BioendoTM Endotoxin Test Kit contains Chromogenic Amebocyte Lysate, Reconstitution Buffer, CSE, Water for BET. Endotoxin detection with Kinetic Chromogenic method requires a kinetic incubating microplate reader such as ELx808IULALXH.
2. Product Parameter
Assay Range: 0.005 - 50EU/ml; 0.001 - 10EU/ml
3. Product Feature and Application
BioendoTM KC Endotoxin Test Kit (Kinetic Chromogenic Assay) features strong resistance to interference, and has advantages of kinetic turbidimetric and end-point chromogenic method. It is especially suitable for endotoxin detection of biological samples like vaccine, antibody, protein, nucleic acid, etc..
Note:
Lyophilized Amebocyte Lysate reagent manufactured by Bioendo is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).

































-228x228.png)
