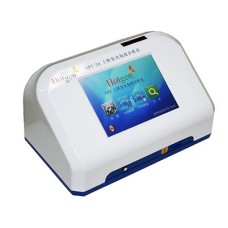
Principle
up-converting phospher technology (UPT) is a kind of immune detection technology that combine immunochromatograpic principle with photoelectric conversion principle.it uses up-converting phosphor particles (ucps) as tracer, after being excited by infrared light,the UCP’s emit visible light, achieving the up-conversion of energy. then get quantitative detection of the test sample rapidly via the conversion of light signals.
Features
- Unique “Up-converting Phosphor” Technology.
- Luminescence method, Fully quantitative.
- Reagent stored at 4~30°C, no need of refrigeration.
- Easy Operation.
- Rapid, get results in 3~20 minutes.
- Multi-item detection.
- Single or bulk test(can reach 100 Tests/Hour).
- Compact Analyzer
- Accurate results can be comparable with the Central Lab.
- Support print results directly or upload to the Hospital LIS system.
UPT (UTP-3A) Procalcitonin Kit
80 Procalcitonin is an accurate, rapid, fully quantitative test used on 80 Reader for the determination of the procalcitonin (PCT) in human serum, plasma and whole blood as an aiding tool for diagnosing and controlling the treatment of severe, bacterial infection and sepsis.
PCT: A specific marker of severe bacterial infection and sepsis
Procalcitonin (PCT) is a peptide precursor of the hormone calcitonin, the latter being involved with calcium homeostasis. It is composed of 116 amino acids and is produced by parafollicular cells (C cells) of the thyroid and by the neuroendocrine cells of the lung and the intestine.
The level of PCT in the blood stream of healthy individuals is below the limit of detection (0.01 ng/mL) of clinical assays. The level of procalcitonin rises rapidly (within 6 – 12 hours) in a response to a proinflammatory stimulus, especially of bacterial origin. It does not rise significantly with viral or non-infectious inflammations. Therefore, PCT helps differentiate bacterial from viral infections, the early detection of an elevated PCT level in patients with suspected bacterial infections enabling earlier antibiotic treatment. PCT also supports informed decisions on when to continue or stop antibiotics, improving patient care and decreasing antibiotic misuse and resistance.
Benefits of PCT test in summary:
Use as an early and specific biomarker for severe bacterial infection and sepsis
Assess severity of infection and make prognosis
Differentiate bacterial from viral and non-infectious inflammation
Guide the application of antibiotics and reduce unnecessary usage of antibiotics
Monitor treatment efficacy of antibiotics
80 Procalcitonin Kit Test Principle
Up-converting Phosphor Technology
80 Procalcitonin Kit Features
- Fully quantitative measurement
- Rapid, accurate, lab quality results within minutes
- Ready-to-use reagents
- Serum, plasma and whole blood are applicable as sample
- Long shelf life
- Easy storage at room temperature
- Single dose format: run only the test you need
UPT (UTP-3A) C-Reactive Protein (CRP) Kit
UPT (UTP-3A)® CRP is an accurate, high-sensitive rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of C-Reactive Protein and hs-CRP in human serum, plasma and blood as a marker of inflammation as well as a diagnostic tool for risk stratification of heart disease.
CRP: the classical acute-phase reactant and an early, non-specific marker for inflammation
The C-Reactive Protein (CRP) is synthesized by the liver in response to interleukin-6 and well known as one of the classical acute-phase reactants and as a marker of inflammation.
CRP is the first acute-phase protein to be described and is an exquisitely sensitive systemic marker of inflammation and tissue damage.
CRP is a more sensitive and accurate reflection of the acute phase response than the ESR. ESR may be normal and CRP elevated. CRP returns to normal more quickly than ESR in response to therapy.
Measuring and charting CRP values can prove useful in determining disease progress or the effectiveness of treatments.
A significantly increased CRP result may indicate the need for immediate antibiotic treatment. The CRP level decreases rapidly in response to effective therapy.
A normal or moderately increased CRP may support a diagnosis of viral or self-limiting infection, which provides valuable information to prevent unnecessary antibiotic prescriptions.
hs-CRP: the new cardiac marker for risk stratification of cardiovascular disease
A high-sensitivity CRP (hs-CRP) test may be used by itself, in combination with other cardiac risk markers, or in combination with a lipoprotein-associated phospholipase A2 (Lp-PLA2) test that evaluates vascular inflammation. The hs-CRP test accurately detects low concentrations of C-reactive protein to help predict a healthy person’s risk of cardiovascular disease (CVD).
High-sensitivity CRP is promoted by some as a test for determining a person’s risk level for CVD, heart attacks, and strokes. The current thinking is that hs-CRP can play a role in the evaluation process before a person develops one of these health problems.
A high hs-CRP of 3 milligrams per liter or above indicates a serious risk of cardiovascular disease. An hs-CRP of 1 to 3 milligrams per liter constitutes an average risk, and an hs-CRP below 1 milligram per liter indicates a low risk of heart disease.
UPT (UTP-3A)® CRP Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® CRP Kit Features
Fully quantitative measurement
Measuring range cover hs-CRP and routine CRP from 0.5 – 150mg/L
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma, blood are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT (UTP-3A) Interleukin 6 (IL-6) Kit
UPT (UTP-3A)® IL-6 is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of interleukin 6 (IL-6) in human serum and plasma as an aiding tool for early diagnosis of infection.
IL-6: An early, non-specific marker for infection
Interleukin 6 (IL-6) is an interleukin that acts as both a pro-inflammatory cytokine and an anti-inflammatory myokine. In humans, it is encoded by the IL6 gene.
IL-6 is secreted by T cells and macrophages to stimulate immune response, e.g. during infection and after trauma, especially burns or other tissue damage leading to inflammation. IL-6 also plays a role in fighting infection, as IL-6 has been shown in mice to be required for resistance against bacterium Streptococcus pneumoniae.
In addition, osteoblasts secrete IL-6 to stimulate osteoclast formation. Smooth muscle cells in the tunica media of many blood vessels also produce IL-6 as a pro-inflammatory cytokine. IL-6’s role as an anti-inflammatory cytokine is mediated through its inhibitory effects on TNF-alpha and IL-1, and activation of IL-1ra and IL-10.
Elevated IL-6 levels may occur in different conditions including and not limited to: chronic infections, autoimmune disorders, certain cancers, and Alzheimer’s disease.
IL-6 test is used to help evaluate a person who has a condition associated with inflammation, such as lupus or rheumatoid arthritis, or with infection, such as sepsis. It may also be used in the evaluation of diabetes or cardiovascular disease.
UPT (UTP-3A)® IL-6 Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® IL-6 Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT (UTP-3A) Lp-PLA2 Kit
UPT (UTP-3A)® Lp-PLA2 is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of Lipoprotein Associated Phospholipase A2 (Lp-PLA2) in human serum and plasma as a risk marker for cardiovascular disease.
Lp-PLA2: the independent risk marker for cardiovascular disease
Lipoprotein-associated phospholipase A2 (Lp-PLA2) is also known as platelet-activating factor acetylhydrolase (PAF-AH). Lp-PLA2 is an enzyme that appears to play a role in the inflammation of blood vessels and is thought to help promote atherosclerosis.
Some recent studies have shown that Lp-PLA2 is an independent risk marker for cardiovascular disease (CVD), including coronary heart disease (CHD), and ischemic stroke. The Lp-PLA2 test is thus used to help evaluate a person’s risk of developing coronary heart disease (CHD) or to help determine the risk of having an ischemic stroke.
The test would typically be used to evaluate an individual who is at a moderate to elevated risk for CHD or stroke, someone with one or more other risk factors. For instance, it may be ordered when someone has normal or minimally elevated lipid levels, borderline high blood pressure (hypertension), or metabolic syndrome.
An Lp-PLA2 test may sometimes be used along with an hs-CRP test to evaluate a person’s level of underlying inflammation associated with CVD risk. However, unlike hs-CRP, the Lp-PLA2 test is not affected by conditions other than CVD that can cause general inflammation, so it may be used when someone has an inflammatory condition, such as arthritis.
UPT (UTP-3A)® Lp-PLA2 Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® Lp-PLA2 Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT (UTP-3A) NT-proBNP Kit
UPT (UTP-3A)® NT-proBNP is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of the N-terminal fragment of brain-type natriuretic peptide in human serum, plasma and whole blood as an aiding tool for screening, diagnosis and risk assessment of life-threatening heart failure (HF).
NT-proBNP: A proven biomarker for heart failure
The N-terminal pro B-type natriuretic peptide (NT-proBNP) is a 76 amino acid N-terminal inactive protein that is cleaved from proBNP to release brain natriuretic peptide.
The plasma concentrations of both BNP and NT-proBNP are typically increased in patients with asymptomatic or symptomatic left ventricular dysfunction and is associated with coronary artery disease and myocardial ischemia. As the latter is more stable and has higher concentration and a longer shelf life in blood, NT-proBNP is a much better biomarker and used much more often.
Early diagnosis of heart failure is key to improve patient outcome. Using NT-proBNP can help clinicians optimize management of patients with dyspnea in the emergency room. This has shown to result in significant cost savings for healthcare structures due to shorter stays in the emergency department and reduced patient re-hospitalization.
International guidelines recommend the use of B-type natriuretic peptide testing in the diagnostic workup of Heart Failure (HF) in both acute and non-acute patient presentation.
Benefits of NT-proBNP test in summary:
Diagnosis of patients with suspected CHF
Risk stratification of patients with HF
Risk stratification of patients with ACS
Assessment of increased risk for cardiovascular events and mortality in patients at risk for HF who have stable coronary artery disease (CAD)
UPT (UTP-3A)® NT-proBNP Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® NT-proBNP Kit Features
Fully quantitative NT-proBNP measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma and whole blood are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT (UTP-3A) Heart-type Fatty Acid Binding Protein (H-FABP) Kit
UPT (UTP-3A)® H-FABP is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of Fatty Acid Binding Protein (H-FABP) in human serum and plasma as an aiding tool for early diagnosis of cardiac infarction.
H-FABP: An early, specific marker for cardiac infarction
Heart-type Fatty Acid-Binding Protein (H-FABP) is a small cytoplasmic protein (15 kDa) released from cardiac myocytes very rapidly following Acute Myocardial Infarction (AMI).
The diagnostic potential of the biomarker H-FABP for heart injury was discovered in 1988 by Professor Jan Glatz (Maastricht, Netherlands). H-FABP concentrations typically peak at approximately 6-8 hours after onset of chest pain and return to normal within 24-30 hours. Although H-FABP has similar release kinetics to Myoglobin (i.e. rapid release & clearance), H-FABP is 20 times more specific to cardiac muscle than myoglobin, it is found at 10-fold lower levels in skeletal muscle than heart muscle and the amounts in the kidney, liver and small intestine are even lower again, hence making it a vastly more efficacious biomarker for myocardial injury.
Furthermore, H-FABP has been repeatedly shown to be a highly sensitive early rise biomarker across the full spectrum of acute coronary syndrome (ACS), detectable as early as 30 minutes following the onset of an ischemic episode.
H-FABP: Release Kinetics
A secondary benefit of the H-FABP release kinetics after AMI is that since it returns to baseline concentrations so rapidly (i.e. typically 20-24 hours), it can be used as a bio marker for recurrence of infarction in the days following an initial AMI. This has been a common utility of CK-MB historically, due to its more rapid clearance than with Troponin (3-4 days vs 10-14 days), but H-FABP offers potential use from as early as the second day onward.
In addition to its diagnostic potential, H-FABP also has prognostic value. Alongside D-dimer, NT-proBNP and peak troponin T, studies show that it was the only cardiac biomarker that proved to be a statistically significant predictor of death or MI at one year. This prognostic information was independent of troponin T, ECG and clinical examination.
H-FABP is a very stable protein in-vitro, as studies have shown that serum and plasma samples can be subjected to up to 8 freeze/thaw cycles without the loss of interactivity.
Benefits of H-FABP test in summary:
Early, sensitive, and specific biomarker for MI
Early and sensitive biomarker for ACS
Ideal biomarker for recurrence of heart injury
Independent cardiac biomarker predicting risk of MI
Recommended to be tested in combination with Troponin I to increase the sensitivity and specificity
UPT (UTP-3A)® H-FABP Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® H-FABP Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT (UTP-3A) Cardiac Troponin I (cTnI) Kit
UPT (UTP-3A)® cTnI is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of cardiac troponin I (cTnI) in human serum, plasma and whole blood as an aiding tool for early diagnosis of heart attack.
cTnI: the most specific cardiac marker for AMI
Human troponin I, often denoted as cTnI, is presented in cardiac muscle tissue by a single isoform with molecular weight 23876 Da and it consists of 209 amino acid residues.
For more than 15 years cTnI has been known as a reliable marker of cardiac muscle tissue injury. It is considered to be more sensitive and significantly more specific in diagnosis of the myocardial infarction than Creatin Kinase isoenzyme MM (CK-MB) and myoglobin.
Measurements of cardiac troponin I are used in the diagnosis and treatment of myocardial infarction and as an aid in the risk stratification of patients with acute coronary syndromes with respect to their relative risk of mortality.
For optimal diagnostic usefulness, a cardiac marker should be specific for cardiac tissue, should be rapidly released into the bloodstream with a direct proportional relationship between the extent of myocardial injury and the measured level of the marker, and should persist in blood for a sufficient length of time to provide a convenient diagnostic time window. The cardiac-specific troponins, troponin I (cTnI) and troponin T (cTnT) are considered the biochemical markers of choice in the evaluation of acute coronary syndromes (ACS) including ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, and unstable angina.
Elevated levels of cardiac-specific troponins convey prognostic information beyond that supplied by the patients clinical signs and symptoms, the ECG at presentation, and the pre-discharge exercise test.
The Joint European Society of Cardiology/American College of Cardiology Committee (ESC/ACC) consensus guidelines for diagnosis of MI include observation of the typical rise and fall of cardiac markers with at least one of: ischemic symptoms, development of pathologic Q waves on electrocardiogram (ECG), ST segment elevation or depression in ECG, or coronary artery intervention; troponin is the preferred marker with a cutoff set at or above the 99th percentile of the reference range.
The World Health Organization (WHO) definition for diagnosis of MI requires the presence of two of: unequivocal ECG changes, unequivocal cardiac marker changes, prolonged chest pain.
Currently it is widely practiced in the world that cTnI, CK-MB and myoglobin are co-measured in suspicion of MI. Recently H-FABP has emerged as a very specific and sensitive cardiac biomarker for MI and the measurement of H-FABP is on the rise worldwide.
UPT (UTP-3A)® cTnI Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® cTnI Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma and whole blood are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT (UTP-3A) Myoglobin (MYO) Kit
UPT (UTP-3A)® Myoglobin is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of myoglobin (MYO) in human serum, plasma and urine as an aiding tool for early diagnosis of heart attack and skeletal muscle injury.
Myoglobin: An early, sensitive cardiac marker for AMI
Myoglobin (MYO) is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. In humans, myoglobin is only found in the bloodstream after muscle (skeletal muscle or heart muscle) injury.
Myoglobin is widely used as a cardiac biomarker, along with troponin, CKMB to help diagnose or rule out a heart attack.
Levels of myoglobin start to rise within 2-3 hours of a heart attack or other muscle injury, reach their highest levels within 8-12 hours, and generally fall back to normal within one day. An increase in myoglobin is detectable sooner than troponin, but it is not as specific for heart damage and it will not stay elevated as long as troponin.
Although a negative myoglobin result effectively rules out a heart attack, a positive result must be confirmed by testing for troponin, which is much more specific for diagnosing acute heart infarction (AMI).
Blood samples are drawn on admission and every 2-3 hours for up to 12 hours in those who come to the emergency room with a possible heart attack.
Sometimes, a urine test is ordered to evaluate myoglobin concentrations in those who have had extensive damage to their skeletal muscles (rhabdomyolysis). Blood levels of myoglobin can rise very quickly with severe muscle injury. Urine myoglobin concentrations reflect the degree of muscle injury and, since myoglobin is toxic to the kidneys, reflect the risk of kidney damage.
UPT (UTP-3A)® Myoglobin Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® Myoglobin Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT (UTP-3A) CK-MB Kit
UPT (UTP-3A)® CK-MB is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of creatine kinase isoenzyme MB (CK-MB) in human serum and plasma as an aiding tool for diagnosis of heart attack.
CK-MB: the traditional cardiac marker for heart attack
There are 3 isoenzymes of creatine kinase (CK): CK-BB, CK-MM, and CK-MB. The primary source of CK-MB is myocardium although it is also found in skeletal muscle. CKMB levels increase with myocardial damage. Extreme elevations of CKMB can also be associated with skeletal muscle cell turnover as in polymyositis and to a lesser degree in rhabdomyolysis. It can also be elevated in cases of carbon monoxide poisoning, crush injuries, pulmonary embolism, hypothyroidism, and muscular dystrophy.
Increased CK-MB can usually be detected in someone with a heart attack about 3-6 hours after the onset of chest pain. The level of CK-MB peaks in 12-24 hours and then returns to normal within about 48-72 hours. If there is a second heart attack or ongoing damage, then levels may rise again and/or stay elevated longer.
CK-MB were once the primary tests ordered to detect and monitor heart attacks, but they have now been largely replaced by the troponin test, which is more specific for damage to the heart. If a troponin test is not available, then the CK-MB test is still considered an acceptable substitute. And it is widely practiced around the world that CK-MB is tested in combination with troponin and myoglobin for diagnosis of myocardial injury.
UPT (UTP-3A)® CK-MB Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® CKMB Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT (UTP-3A) D-dimer Kit
UPT (UTP-3A)® D-dimer is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of D-dimer in human plasma as an aiding tool for diagnosing and monitoring thrombotic disorders.
D-dimer: an important prognostic indicator of heart diseases
D-dimer (or D dimer) is a fibrin degradation product (or FDP), a small protein fragment present in the blood after a blood clot is degraded by fibrinolysis. It is so named because it contains two crosslinked D fragments of the fibrin protein.
D-dimer test is primarily used to exclude thromboembolic disease where the probability is low. Since its introduction in the 1990s, it has become an important test performed in patients with suspected thrombotic disorders. While a negative result practically rules out thrombosis, a positive result can indicate thrombosis but does not rule out other potential causes.
D-dimer tests are used to help rule out the presence of an inappropriate blood clot (thrombus). Some of the conditions that the D-dimer test is used to help rule out include:
Deep vein thrombosis (DVT)
Pulmonary embolism (PE)
Stroke
Measurement of the D-Dimer level in plasma has been used as a screening strategy for subclinical DVT. D-Dimer is well known to be an important prognostic indicator of heart diseases and its most definitive role is on monitoring post-treatment clinical status and the post therapeutic evaluation of patients.
A D-dimer level may also be used to help diagnose disseminated intravascular coagulation (DIC) and to monitor the effectiveness of DIC treatment.
UPT (UTP-3A)® D-dimer Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® D-dimer Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT (UTP-3A) NGAL Kit
UPT (UTP-3A)® NGAL is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of neutrophil gelatinase-associated lipocalin (NGAL) in human serum, plasma and urine as an aiding tool for diagnosing and controlling the treatment of acute kidney injury (AKI).
NGAL: A novel and early, sensitive biomarker for AKI
NGAL (neutrophil gelatinase-associated lipocalin, lipocalin-2, siderocalin) is a small protein expressed in neutrophils and certain epithelia, including the renal tubules. Renal expression of NGAL is dramatically increased in kidney injury from a variety of causes, and NGAL is released into both urine and plasma. NGAL levels rise within 2 hours of the insult, making NGAL an early and sensitive biomarker of kidney injury.
In the case of acute kidney injury (AKI), NGAL is secreted in high levels into the blood and urine within 2 hours of injury. Because NGAL is protease resistant and small, the protein is easily excreted and detected in the urine. NGAL levels in patients with AKI have been associated with the severity of their prognosis and can be used as a biomarker for AKI. NGAL can also be used as an early diagnosis for procedures such as chronic kidney disease, contrast induced nephropathy, and kidney transplant.
Kidney health is most frequently measured by serum creatinine. Serum creatinine is a marker of kidney function, whereas NGAL is a marker of kidney injury. NGAL levels are a more precise and sensitive marker for diagnosing AKI than serum creatinine levels. In fact, the increase in urinary excretion of NGAL has been proven to be due to tubular alterations that take place before any damage can be detected by other methods Therefore, monitoring NGAL levels reduces delayed AKI diagnosis and treatment. Using a more sensitive and specific marker allows for earlier diagnosis, correct responses to AKI, and reduced risk of morbidity and mortality
Benefits of NGAL test in summary:
Early diagnosis of AKI to allow earlier initiation of appropriate management
Risk stratification of AKI
Prediction of clinical outcomes (dialysis, in-hospital death, length of hospital stay, mortality)
Monitor response to therapy
Lower hospitalization costs
UPT (UTP-3A)® NGAL Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® NGAL Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT(UTP-3A) Fetal Fibronectin (fFN) Kit
UPT (UTP-3A)® fFN is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of Fetal Fibronectin (fFN) in human cervicoviginal secretions as a tool for risk estimation of preterm labor.
fFN: A specific biomarker for risk estimation of preterm delivery
Fetal Fibronectin (fFN) is a protein that acts as a “glue” during pregnancy, attaching amniotic sac to the lining of uterus. Disruption of this interface causes the release of fFN into cervical/vaginal secretions.
As a new-found specific biomarker for preterm delivery, fFN is highly associated with risk of preterm delivery when present in cervicovaginal secretions.
fFN testing is approved by FDA for use in women from weeks 22 to 35 of pregnancy.
A positive fFN test result indicates that a woman have high risk of preterm labor, although it can’t tell for sure that she will go into labor soon. A negative result means that preterm delivery will 98% unlikely happen to the pregnant woman within one to two weeks, and the test can be repeated weekly for women who remain at high risk.
Benefits of fFN test in summary:
Specific biomarker for risk assessment of preterm labor in women from 22 to 35 weeks of pregnancy
Positive result indicates high risk of preterm delivery
Negative result can almost rule out the possibility of preterm delivery within two weeks
Help avoid unnecessary intervention and hospitalizations
UPT (UTP-3A)® fFN Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® fFN Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT(UTP-3A) Anti-CCP Kit
UPT (UTP-3A)® Anti-CCP is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of Anti-Cyclic Citrullinated Peptide antibody in human serum and plasma as an aiding tool for diagnosing and controlling the treatment of rheumatoid arthritis.
Anti-CCP: An early, specific marker for rheumatoid arthritis (RA)
Anti-CCP antibodies are highly sensitive and specific for Rheumatoid Arthritis (RA). Studies show Anti-CCP test demonstrates an outstanding sensitivity of over 80% and a specificity of over 96%. The presence of anti-CCP antibodies can also be used to predict which patients will get more severe rheumatoid arthritis. The anti-CCP test enables clinicians to effectively distinguish RA patients from other RA-resembling diseases, even in cases where the rheumatoid factor (RF) is not discriminative. Anti-CCP antibodies can be detected very early in the course of RA.
The presence of anti-CCP is associated with significantly higher levels of erosions compared to rheumatoid factor and other parameters. Anti-CCP is an independent predictor of radiological damage and progression.
In 2009, Anti-CCP antibodies were recommended as diagnostic biomarker for early detection of rheumatoid arthritis in an ACR(American College of Rheumatology) meeting.
Benefits of Anti-CCP test in summary:
Use as an early and specific biomarker for rheumatoid arthritis
Demonstrate greater specificity than RF while maintaining or increasing sensitivity
Differentiate erosive from less or non-erosive RA
Help physicians decide when aggressive therapy with biologicals is needed
Monitor the treatment efficacy of drugs for RA
UPT (UTP-3A)® Anti-CCP Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® Anti-CCP Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT(UTP-3A) alpha-Fetoprotein (AFP) Kit
UPT (UTP-3A)® Anti-CCP is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of Anti-Cyclic Citrullinated Peptide antibody in human serum and plasma as an aiding tool for diagnosing and controlling the treatment of rheumatoid arthritis.
Anti-CCP: An early, specific marker for rheumatoid arthritis (RA)
Anti-CCP antibodies are highly sensitive and specific for Rheumatoid Arthritis (RA). Studies show Anti-CCP test demonstrates an outstanding sensitivity of over 80% and a specificity of over 96%. The presence of anti-CCP antibodies can also be used to predict which patients will get more severe rheumatoid arthritis. The anti-CCP test enables clinicians to effectively distinguish RA patients from other RA-resembling diseases, even in cases where the rheumatoid factor (RF) is not discriminative. Anti-CCP antibodies can be detected very early in the course of RA.
The presence of anti-CCP is associated with significantly higher levels of erosions compared to rheumatoid factor and other parameters. Anti-CCP is an independent predictor of radiological damage and progression.
In 2009, Anti-CCP antibodies were recommended as diagnostic biomarker for early detection of rheumatoid arthritis in an ACR(American College of Rheumatology) meeting.
Benefits of Anti-CCP test in summary:
Use as an early and specific biomarker for rheumatoid arthritis
Demonstrate greater specificity than RF while maintaining or increasing sensitivity
Differentiate erosive from less or non-erosive RA
Help physicians decide when aggressive therapy with biologicals is needed
Monitor the treatment efficacy of drugs for RA
UPT (UTP-3A)® Anti-CCP Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® Anti-CCP Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT(UTP-3A) Golgi Protein 73 (GP73) Kit
UPT (UTP-3A)® GP73 is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of golgi protein 73 in human serum and plasma as an aiding tool for diagnosis and risk stratification of hepatocellular carcinoma.
GP73: a novel and highly specific marker for hepatocellular carcinoma
Golgi protein 73 (GP73, also known as Golph2), is a resident Golgi-specific membrane protein expressed by biliary epithelial cells in normal liver, and its expression is increased markedly in chronic liver diseases, especially in HCC cells.
Alpha-fetoprotein (AFP), together with hepatic ultrasonography, is the most common marker used in clinical practice to detect HCC in cirrhotic patients, and has been considered the gold-standard serum marker for screening patients at high risk for HCC, as well as for the diagnosis and monitoring of responses to HCC treatment for over 40 years. But the clinical value of AFP is challenged in recent years due to low sensitivity and specificity. In addition, AFP levels greater than 500 ng/ml are correlated with the tumor size: 80% of small HCC show no increase of AFP concentration. Some patients with cirrhosis and/or hepatic inflammation could have an elevated level of AFP without the presence of tumors. Therefore, serum markers with better diagnostic accuracy are needed for HCC.
Studies indicate that serum GP73 has a comparable accuracy to AFP for the diagnosis of HCC. The summary of 8 clinical studies estimates for serum GP73 and AFP in diagnosing HCC were as follows: sensitivity, 76% (95% confidence interval (CI) 51-91%) vs. 70% (47-86%); specificity, 86% (95%CI 65-95%) vs. 89% (69-96%); diagnostic odds ratio (DOR), 18.59 (95%CI 5.33-64.91) vs. 18.00(9.41-34.46); and area under sROC, 0.88 (95%CI 0.77-0.99) vs. 0.86 (95%CI 0.84-0.87).
UPT (UTP-3A)® GP73 Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® GP73 Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT(UTP-3A) TIMP-1 Kit
UPT (UTP-3A)® TIMP-1 is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of Metallopeptidase Inhibitor 1 (TIMP-1) in human serum and plasma as an aid in the diagnosis, monitoring and prognosis of hepatic fibrosis and cirrhosis.
TIMP-1: novel marker for liver fibrosis
Matrix MetalloProteinases (MMPs) are zinc-dependent endopeptidases that catalyze degradation of extracellular matrix proteins, thereby controlling such processes as development, tissue remodeling, wound healing and tumor metastasis. The activity of MMPs is controlled by regulation of expression and secretion, by proteolytic activation of pro-enzymes and by the Tissue Inhibitors of MetalloProteinases (TIMPs).
TIMP-1 is a 184 amino-acid residue glycosylated protein and is widely synthesized by many cells and tissues. Many physiological functions of TIMP-1 are closely tied to the functions of MMPs, and an improper balance of MMP and TIMP production correlates with pathological conditions such as arthritis, tumor growth and metastasis. On the other hand, TIMP-1 was independently discovered as an erythroid potentiating activity, an activity that appears to be functionally distinct from MMP inhibitory activity. TIMP-1 binds to certain cell lines and is translocated to the nucleus. It inhibits apoptosis in B-cells, further suggesting that TIMP-1 independently functions in multiple ways to support survival and growth of cells in contrast to its function of inhibition MMP.
Early diagnosis of liver fibrosis is key for preventing chronic hepatitis progressing to life-threatening cirrhosis
Currently, there are hundreds of million patients with liver fibrosis throughout the world, and most cases are caused by hepatitis viral infections. Viral hepatitis, hepatic cirrhosis, and hepatocellular carcinoma are considered to be the three steps of liver disease.
Hepatic cirrhosis is a progressive consequence of liver fibrosis. Liver fibrosis refers to the accumulation of extracellular matrix (ECM) proteins, which occurs in most types of chronic diseases. Of these proteins, collagen, which is the body’s self-repairing protein induced by inflammation, most commonly accumulates. The formation and progression of liver fibrosis is an extremely complicated, gradual process, which is mainly reflected as sustainable inflammation in the liver inducing an ecological disequilibrium of cells and intracellular substances.
To prevent liver fibrosis from progressing to liver cirrhosis, early detection of fibrosis and timely treatment are most important. Only in this way can we control the progression of chronic hepatitis into liver fibrosis and hepatic cirrhosis.
HA,LN, PIIINP, CIV, TIMP-1: the laboratory test panel that has the potential of replacing the invasive biopsy examination for liver fibrosis and liver cirrhosis
Liver biopsy has been regarded as gold standard method for the diagnosis of liver fibrosis. But it has many limitations, such as pain, injury to the body, sampling error and individual variation in interpreting the results.
Currently, serum liver fibrosis markers have been employed as non-invasive diagnosis of liver fibrosis and evaluation of the severity of liver fibrosis and cirrhosis. They include laminin (LN), hyaluronic acid (HA), collagen type IV (CIV), N-terminal propeptide of collagen III (PIIINP) and metallopeptidase inhibitor 1 (TIMP-1).
Studies show that blood level of one or all the markers of LN, HA, CIV, PIIINP and TIMP-1 increases during the progression of chronic hepatitis to liver fibrosis or further to liver cirrhosis. A combination of the five tests would be clinically more useful for predicting significant fibrosis in patients with chronic hepatitis B and C, when liver biopsy is contraindicated.
UPT (UTP-3A)® TIMP-1 Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® TIMP-1 Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT(UTP-3A) PIIINP Kit
UPT (UTP-3A)® CIV is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of N-Terminal Peptide of Procollagen III (PIIINP) in human serum and plasma as an aid in the diagnosis, monitoring and prognosis of hepatic fibrosis and cirrhosis.
PIIINP – biomarker for non-invasive diagnosis of liver fibrosis
Increased concentrations of N-terminal peptide of type III procollagen (PIIINP) are found in a number of conditions where accumulation and/or degradation of connective tissue takes place, e.g. in fibroproliferative, haematological, endocrinological and malignant diseases. Thus changes in PIIINP levels are not specific for a particular disease but reflect the involvement and altered metabolism of type III collagen. In growth deficient children, the induction of growth is reflected in increasing serum PIIINP. Liver fibrosis and cirrhosis of various aetiologies increase serum concentration of PIIINP. Serum PIIINP is increased in myeloproliferative diseases, particularly during the active phases of myelofibrosis. The serum concentration of PIIINP also appears to reflect the repair process and scar formation after a myocardial infarction.
Early diagnosis of liver fibrosis is key for preventing chronic hepatitis progressing to life-threatening cirrhosis
Currently, there are hundreds of million patients with liver fibrosis throughout the world, and most cases are caused by hepatitis viral infections. Viral hepatitis, hepatic cirrhosis, and hepatocellular carcinoma are considered to be the three steps of liver disease.
Hepatic cirrhosis is a progressive consequence of liver fibrosis. Liver fibrosis refers to the accumulation of extracellular matrix (ECM) proteins, which occurs in most types of chronic diseases. Of these proteins, collagen, which is the body’s self-repairing protein induced by inflammation, most commonly accumulates. The formation and progression of liver fibrosis is an extremely complicated, gradual process, which is mainly reflected as sustainable inflammation in the liver inducing an ecological disequilibrium of cells and intracellular substances.
To prevent liver fibrosis from progressing to liver cirrhosis, early detection of fibrosis and timely treatment are most important. Only in this way can we control the progression of chronic hepatitis into liver fibrosis and hepatic cirrhosis.
HA,LN, PIIINP, CIV, TIMP-1: the laboratory test panel that has the potential of replacing the invasive biopsy examination for liver fibrosis and liver cirrhosis
Liver biopsy has been regarded as gold standard method for the diagnosis of liver fibrosis. But it has many limitations, such as pain, injury to the body, sampling error and individual variation in interpreting the results.
Currently, serum liver fibrosis markers have been employed as non-invasive diagnosis of liver fibrosis and evaluation of the severity of liver fibrosis and cirrhosis. They include laminin (LN), hyaluronic acid (HA), collagen type IV (CIV), N-terminal propeptide of collagen III (PIIINP) and metallopeptidase inhibitor 1 (TIMP-1).
Studies show that blood level of one or all the markers of LN, HA, CIV, PIIINP and TIMP-1 increases during the progression of chronic hepatitis to liver fibrosis or further to liver cirrhosis. A combination of the five tests would be clinically more useful for predicting significant fibrosis in patients with chronic hepatitis B and C, when liver biopsy is contraindicated.
UPT (UTP-3A)® PIIINP Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® PIIINP Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT(UTP-3A) Collagen IV (CIV) Kit
UPT (UTP-3A)® CIV is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of collagin IV (CIV) in human serum and plasma as an aid in the diagnosis, monitoring and prognosis of hepatic fibrosis and cirrhosis.
Collagen IV – Biomarker for non-invasive diagnosis of liver fibrosis
Collagen IV (ColIV or Col4) is a type of collagen found primarily in the basal lamina. Liver fibrosis and cirrhosis are associated with the deposition of collagen IV in the liver. Studies found that serum Collagen IV concentrations correlate with hepatic tissue levels of collagen IV in subjects with alcoholic liver disease and viral hepatitis and fall following successful therapy.
The formation of hepatic fibrosis is resulting from the over produce or deficient degradation, or both, of the extracellular matrix, hence excessive connective tissue builds up in the liver, then fibrosis is in turn formed. In a normal liver, type IV collagen concentration is very low. During the fibrosis process from hepatitis through to cirrhosis, the formation of basal membrane occurs, and type IV collagen concentration in liver tissue and blood stream increases accordingly. So CIV serological tests are able to identify different stages of liver fibrosis with relative accuracy, which indicates various changes during the development of liver fibrosis. Additionally, the effects of anti-fibrosis therapies could be monitored with a CIV assay kit by determine the level of CIV.
Early diagnosis of liver fibrosis is key for preventing chronic hepatitis progressing to life-threatening cirrhosis
Currently, there are hundreds of million patients with liver fibrosis throughout the world, and most cases are caused by hepatitis viral infections. Viral hepatitis, hepatic cirrhosis, and hepatocellular carcinoma are considered to be the three steps of liver disease.
Hepatic cirrhosis is a progressive consequence of liver fibrosis. Liver fibrosis refers to the accumulation of extracellular matrix (ECM) proteins, which occurs in most types of chronic diseases. Of these proteins, collagen, which is the body’s self-repairing protein induced by inflammation, most commonly accumulates. The formation and progression of liver fibrosis is an extremely complicated, gradual process, which is mainly reflected as sustainable inflammation in the liver inducing an ecological disequilibrium of cells and intracellular substances.
To prevent liver fibrosis from progressing to liver cirrhosis, early detection of fibrosis and timely treatment are most important. Only in this way can we control the progression of chronic hepatitis into liver fibrosis and hepatic cirrhosis.
HA,LN, PIIINP, CIV, TIMP-1: the laboratory test panel that has the potential of replacing the invasive biopsy examination for liver fibrosis and liver cirrhosis
Liver biopsy has been regarded as gold standard method for the diagnosis of liver fibrosis. But it has many limitations, such as pain, injury to the body, sampling error and individual variation in interpreting the results.
Currently, serum liver fibrosis markers have been employed as non-invasive diagnosis of liver fibrosis and evaluation of the severity of liver fibrosis and cirrhosis. They include laminin (LN), hyaluronic acid (HA), collagen type IV (CIV), N-terminal propeptide of collagen III (PIIINP) and metallopeptidase inhibitor 1 (TIMP-1).
Studies show that blood level of one or all the markers of LN, HA, CIV, PIIINP and TIMP-1 increases during the progression of chronic hepatitis to liver fibrosis or further to liver cirrhosis. A combination of the five tests would be clinically more useful for predicting significant fibrosis in patients with chronic hepatitis B and C, when liver biopsy is contraindicated.
UPT (UTP-3A)® CIV Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® CIV Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT(UTP-3A) Laminin (LN) Kit
UPT (UTP-3A)® LN is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of laminin (LN) in human serum and plasma as an aid in the diagnosis, monitoring and prognosis of hepatic fibrosis and cirrhosis.
Laminin – biomarker for non-invasive diagnosis of hepatic fibrosis and cirrhosis
Laminin is a large (900 kDa) complex extracellular glycoprotein and a major component of the basement membrane. Laminin is thought to mediate the attachment, migration, and organization of cells into tissues during embryonic development by interacting with other extracellular matrix components.
Laminin is composed of three different polypeptide chains; alpha, beta and gamma which are bound to each other by disulphide bonds into a cross-shaped molecule. LN is mainly found in lipocytes, hepatocytes and endothelial cells. It functions with type IV collagen to form basement membrane zone and to stabilize it.
With the development of hepatic fibrosis and cirrhosis, LN is synthesized more quickly and deposits within Disse gaps. The binding with type IV collagen forms a continuous basement membrane, influencing the exchange of nutrients and metabolites between blood and tissues and causing hepatocyte failures. Meanwhile, it might be the major material basis for portal vein hypertension. When connective tissue builds up in the liver, then fibrosis is in turn formed.
Hepatic fibrosis is the common pathological basis for chronic liver disorders. From scientific findings, the worse the liver functions of cirrhosis patients are, the higher the serum LN concentrations would be. Therefore, laminin is a marker of the progression of hepatic sinusoid capillarization and portal liver fibrosis.
Early diagnosis of liver fibrosis is key for preventing chronic hepatitis progressing to life-threatening cirrhosis
Currently, there are hundreds of million patients with liver fibrosis throughout the world, and most cases are caused by hepatitis viral infections. Viral hepatitis, hepatic cirrhosis, and hepatocellular carcinoma are considered to be the three steps of liver disease.
Hepatic cirrhosis is a progressive consequence of liver fibrosis. Liver fibrosis refers to the accumulation of extracellular matrix (ECM) proteins, which occurs in most types of chronic diseases. Of these proteins, collagen, which is the body’s self-repairing protein induced by inflammation, most commonly accumulates. The formation and progression of liver fibrosis is an extremely complicated, gradual process, which is mainly reflected as sustainable inflammation in the liver inducing an ecological disequilibrium of cells and intracellular substances.
To prevent liver fibrosis from progressing to liver cirrhosis, early detection of fibrosis and timely treatment are most important. Only in this way can we control the progression of chronic hepatitis into liver fibrosis and hepatic cirrhosis.
HA,LN, PIIINP, CIV, TIMP-1: the laboratory test panel that has the potential of replacing the invasive biopsy examination for liver fibrosis and liver cirrhosis
Liver biopsy has been regarded as gold standard method for the diagnosis of liver fibrosis. But it has many limitations, such as pain, injury to the body, sampling error and individual variation in interpreting the results.
Currently, serum liver fibrosis markers have been employed as non-invasive diagnosis of liver fibrosis and evaluation of the severity of liver fibrosis and cirrhosis. They include laminin (LN), hyaluronic acid (HA), collagen type IV (CIV), N-terminal propeptide of collagen III (PIIINP) and metallopeptidase inhibitor 1 (TIMP-1).
Studies show that blood level of one or all the markers of LN, HA, CIV, PIIINP and TIMP-1 increases during the progression of chronic hepatitis to liver fibrosis or further to liver cirrhosis. A combination of the five tests would be clinically more useful for predicting significant fibrosis in patients with chronic hepatitis B and C, when liver biopsy is contraindicated.
UPT (UTP-3A)® LN Kit Test Principle
Up-converting Phosphor Technology
UPT (UTP-3A)® LN Kit Features
Fully quantitative measurement
Rapid, accurate, lab quality results within minutes
Ready-to-use reagents
Serum, plasma are applicable as sample
Long shelf life
Easy storage at room temperature
Single dose format: run only the test you need
UPT(UTP-3A) Hyaluronic Acid (HA) Kit
UPT (UTP-3A)® HA is an accurate, rapid, fully quantitative test used on UPT (UTP-3A)® Reader for the determination of hyaluronic acid (HA) in